Abstract
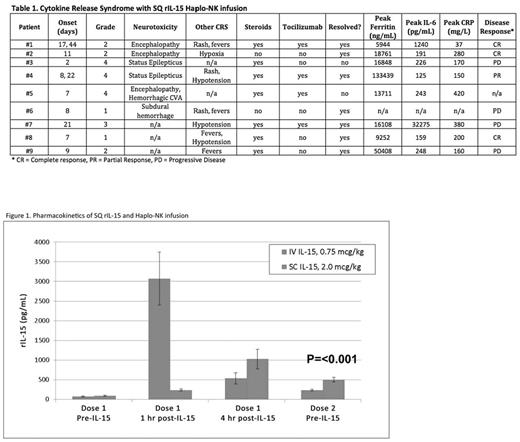
Cytokine Release Syndrome (CRS) is associated with elevated cytokine levels, macrophage activation, and IL-6 production after antibody or cell-based immunotherapy. We have observed over 200 patients with no CRS following adoptively transferred haploidentical donor natural killer (haplo-NK) cell products after lymphodepleting chemotherapy plus IL-2; and achieved complete remission in 30-50% of patients with refractory AML. To promote NK cell expansion without stimulation of Treg that may compromise NK function, we tested the substitution of IL-2 with the NCI manufactured rIL-15. Using the same lymphodepleting preparative regimen (Fludarabine 25 mg/m2IV for 5 days and Cyclophosphamide 60 mg/kg IV for 2 days) we infused CD3- and CD19-depleted haplo-NK incubated overnight with rIL-15 (10 ng/mL) prior to infusion. Two cohorts received rIL-15; either intravenously x 12 daily doses (at the Phase I MTD of 0.75 mcg/kg) or subcutaneously x 10 doses (5 days on, 2 days off, 5 days on at 2 mcg/kg SQ, the MTD when used as monotherapy in solid tumor patients). CRS was defined by modified CRS grading criteria ( Lee et al. Blood, 2014). Results: Forty patients with relapsed/refractory AML were treated with haplo-NK plus IV rIL-15 (n=24) or SQ rIL-15 (n=16). Patients received an average of 11.6 IV doses [9-12, 96% of planned doses] or 7.5 SQ doses [1-10, 75% of planned doses]. Dosing was stopped for toxicity. No CRS events were observed after IV dosing. However, after SQ dosing, 9 of 16 patients [56%] had grade 1-4 CRS; [4 (25%) with grade 3-4 CRS) (Table 1). The median time from NK infusion to onset of CRS was 8 days [2-44]. Six of the 9 CRS patients (66%) had neurological events. Neurological toxicities included grade 1-2 encephalopathy (n=2), intracranial hemorrhage (ICH) (n=2, grade 1 and grade 5), and grade 4 seizures (n=2). One additional event (a patient with a fall resulting in ICH due to pre-existing gait abnormality) without evidence of CRS was not considered as IL-15 associated neurotoxicity CRS. Symptoms resolved in 4 of 5 patients who received treatment for neurologic CRS (3 received both steroids and Tocilizumab, 1 received steroids only, 1 patient did not require treatment as symptoms resolved with stopping IL-15). Non-neurologic toxicity included hypoxia (n=2) and hypotension (n=3). No significant correlations were found between development of CRS and absolute lymphocyte or monocyte count, presence or absence of haplo-NK expansion at day 14, number of rIL-15 doses received, or disease burden at baseline as measured by blast percentage in bone marrow. In the IV rIL-15 cohort, only 2 of 14 patients at the MTD (14%) cleared leukemia and underwent transplantation. In contrast, 8 of 16 patients (50%, P=0.04) in the SQ rIL-15 cohort achieved CR (n=7) or CRp (n=1), with 6 patients proceeding to alloHCT consolidation. CRS did not limit the clinical benefit in the SQ cohort as 4 of 9 [44%] patients with CRS achieved CR/PR compared to 4 of 7 [57%] patients without CRS. Pharmacokinetic data showed slower clearance of rIL-15 in SQ compared to IV dosing, as shown by higher trough levels prior to dose 2 (501 vs. 233 pg/mL, p < 0.001; Figure 1). The average peak level of IL-6 was higher with SQ vs. IV IL-15 (375 pg/mL vs. 47 pg/mL, p = 0.03). Traditional biomarkers did not stratify the grade of CRS, as patients with grade 1-2 (n=5) compared to grade 3-4 CRS (n=4) showed similar median serum values of peak Ferritin (14006 vs. 16478 ng/mL), CRP (180 vs. 275 mg/L), and IL-6 (219 vs. 234 pg/mL). This is the first report of CRS and neurologic symptoms in patients receiving haplo-NK adoptive transfer after lymphodepletion using SQ dosing of NCI rIL-15; a toxicity not seen in similar patients treated with SQ IL-2 or IV rhIL-15. Because this same dose of SQ rIL-15 was previously shown to be safe when given as monotherapy (CITN11-02), our data suggest that the lymphodepleting chemotherapy altered IL-15 clearance dynamics. In contrast to CRS associated with CAR-T therapy, higher disease burden was not associated with CRS, development of CRS was not predictive of disease response, and cardiorespiratory manifestations of CRS were less severe. It is unknown whether different IL-15 products may carry this risk when given after lymphodepletion. Thus dosing schemas with other agents should consider the role of lymphodepletion in altering the clearance of total IL-15. Our current protocols now include prospective monitoring of CRS biomarkers and defined treatment guidelines.
Bachanova: Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Seattle-Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Zymogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oxis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Miller: Celegene: Consultancy; Oxis Biotech: Consultancy; Fate Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal